The Noble Gases
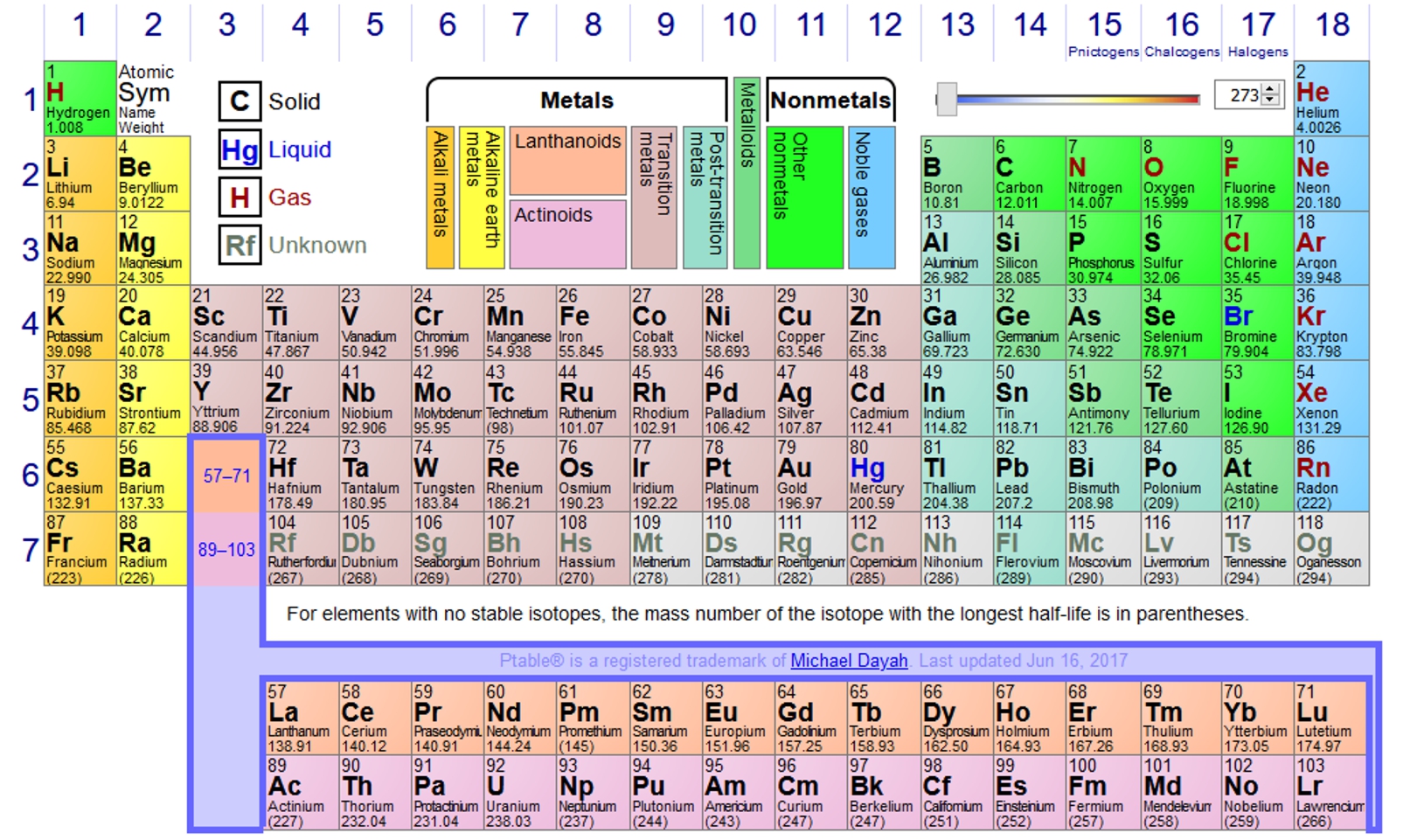
The six naturally occurring noble gases are Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn). They are group 18 in the periodic table.
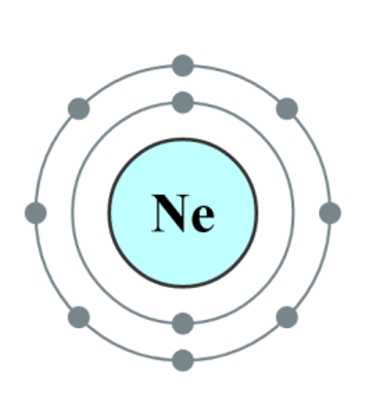
They all share similar properties, being odorless, tasteless and non-flammable. The reason they have such low reactivity is because the outer electron cell is “full”, consequently only a very few noble gas compounds have been formed. In the case of neon, below, both electron shells are complete, and neon is therefore a stable monatomic gas under most conditions.
Helium
Helium is a colourless gas with a boiling point of 4.2K (about -269oC) and density of 0.0002g/cm3 (density of air is 0.0012g/cm3). While helium is the second most abundant element in the universe, it is very rare in the Earth’s atmosphere. It was initially very expensive, until discovered in natural gas wells in Texas, Russia, Poland, Algeria, China and Canada.
It is used to cryogenically freeze biological materials for later use and in welding and other industrial uses. It is added to dive tanks to increase the absorption of oxygen and in cooling nuclear power plants. Finally, because it is so much lighter than the air, it is used in balloons at parties and in blimps.
Neon
Neon is a colourless gas with a boiling point of 27.07K (-246oC) and density of 0.00089g/cm3. It is the fourth most abundant element in the universe, behind, hydrogen, helium and oxygen. It is produced through the liquefaction of air and through separation using fractional distillation.
It is used in television tubes, wave metre tubes and lightning arrestors on telephone poles. When used in vacuum tubes it glows red-orange and is used in advertising. It is also used as a refrigerant and, with helium, in lasers.
Argon
Argon is a colourless gas with a boiling point of 87.3K ( -185oC) and a density of 0.00145g/cm3. It is abundant in the atmosphere at 0.9% by volume and is produced through fractionation of liquid air.
It is primarily used as an inert gas shield in welding and cutting and as a protective blanket in the manufacture of reactive elements such as titanium. It is particularly effective in double glazing and glows blue in vacuum tubes.
Krypton
Krypton is a colourless gas with a boiling point of 119.9K (-153oC) and a density of 0.00375g/cm3. It is the sixth most abundant gas in the atmosphere and is recovered from air by liquefaction and fractionation. It can bond with other elements to form unstable compounds, with the exception of KrF2 which is stable.
It is used in various lighting applications such as airport runway strobe lights and in photography since its light is white. In a vacuum tub, when combined with other gases it produces a yellow light. It is also used in various lasers.
Xenon
Xenon is a rare, colourless heavy gas with a boiling point of 165.0K (-108oC) and a density of 0.00588g/cm3. It is expensive to collect (by fractional distillation) from air where it is less than 1ppm by volume.
It is used in photographic flashes and motion picture arc lamps. It is used in instruments for radiation detection and in medicine as an anaesthetic. It has nine stable isotopes, along with 18 unstable radioactive isotopes.
Radon
Radon is the densest gas known, it is heavier than air and is radioactive. It has a boiling point of 211.5K (-61.7oC) and a density of 0.00907g/cm3. It is produced by the radioactive decay of radium and can occur in spring water and rocks. Because it is so heavy it accumulates at ground level and can be a problem in the basements of homes.
It has medical uses such as in radiation therapy and it has limited scientific uses. Exposure to radon through breathing it can result in lung disease.
An approximation to colours of the various gases in a vacuum tube.
Below a couple of excellent examples of the use of noble gases (combined with some other gases) in advertising.
Indicative Prices
Pricing for noble gases, other than helium, is not transparent. The prices below will only approximate to an actual price, however the price relationship between the gases is realistic.
Helium USD4.29/m3 2019 US non-gov users
Neon USD50-60/m3 2020 Alibaba 1cm3 cylinders
Argon USD68/m3 2020 retail Aust, D Cylinder, 2.1m3
Krypton USD200/m3 2020, GD Special Gas
Xenon USD80-500/m3 2020 Alibaba, price depends on volume
Conclusion
Nobel gases are so named because they very rarely react with anything. They are the most stable of elements, but with limited technological uses. Nonetheless, they are amongst the most impressive of elements.