The Valuable Minerals in Heavy Mineral Sands – Rutile & Leucoxene
Rutile has a theoretical TiO2 content of 100%, however impurities such as iron typically reduce this to 93-97%. TiO2 can also exist as anatase, which has a different crystal structure to rutile. Anatase is primarily used in paper rather than paints and plastics.
Anatase is able to convert light into an electrical charge. This has led to its use in photovoltaics, photo catalysts, and water and air purification.
In 2016 world production of rutile amounted to 743,000t, with the largest producers being Australia, Sierra Leone and Ukraine. According to the United States Geological Survey, world reserves are 59Mt.
The majority of rutile, like ilmenite, is recovered from heavy mineral sand deposits. However, titanium minerals occur in a variety of other settings. They occur in tin and gold placers, and in various hard rock settings.
Rutile is used directly as welding electrode flux or more commonly treated by the chloride process to produce 100% titanium dioxide or, with surface treatment, titanium dioxide pigment. The process oxidises TiO2 then adds chlorine to produce TiCl4. This is then oxidized in a furnace to produce TiO2, the chlorine is recycled.
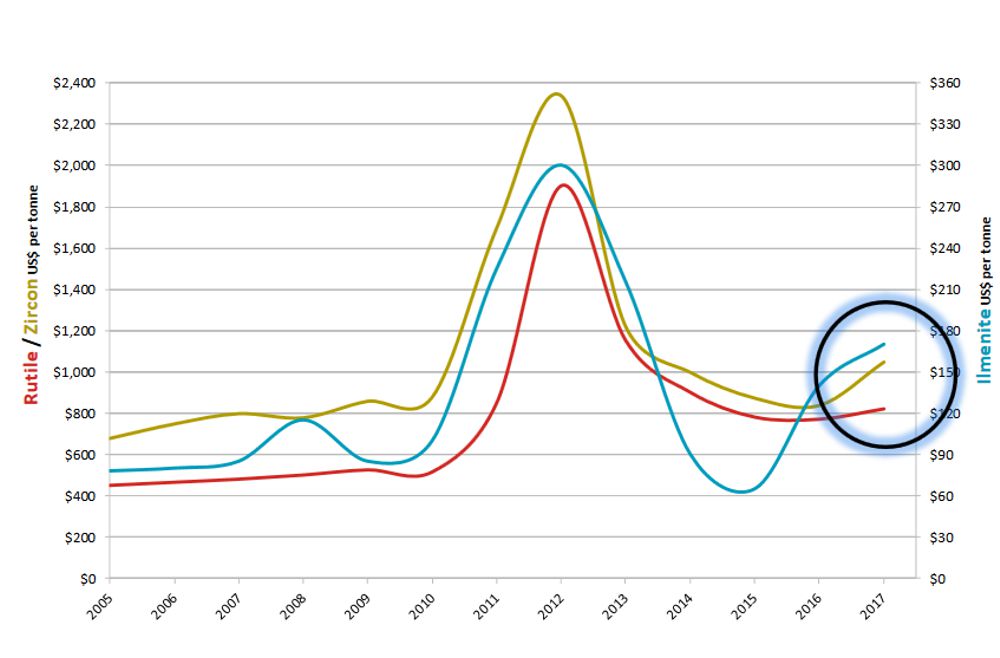
The price of rutile, like those of ilmenite and zircon, has been highly variable over the past decade, as can be seen in the chart below.
Chart Courtesy Base Resources Limited
Leucoxene is formed through the weathering of ilmenite. It does not have a crystal structure and because its composition is highly variable, it does not have a chemical formula. The weathering process removes iron, so leucoxene can be much higher grade than ilmenite, up to 90% TiO2.
Along with rutile, it is used as a feedstock for the chlorite process, and therefore tends to track the rutile price. High grade leucoxene, say 86 to 90% TiO2, will typically command 80-85% of the rutile price.